The world of electrons is a fascinating realm that impacts our daily lives. Understanding these tiny particles reveals just how integral they are to everything around us. From technology to chemistry, the electron plays a critical role. This article will explore the essential components of electron theory, shedding light on its significance and implications.
The Fundamental Properties of Electrons
Charge and Mass: Defining Characteristics and Measured Values
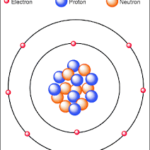
Electron are negatively charged particles. Their charge is measured in coulombs, with each electron holding a precise value of approximately -1.6 x 10^-19 coulombs. In terms of mass, an electron weighs about 9.11 x 10^-31 kilograms. Although incredibly tiny, the characteristics of these particles have vast implications in science.
Wave-Particle Duality: The Electron’s Elusive Nature and the Double-Slit Experiment
A surprising feature of electrons is their wave-particle duality. This means they can act as both particles and waves. The famous double-slit experiment demonstrates this concept. When electrons pass through two slits, they create an interference pattern, typical of waves. However, if observed, they behave like particles, showcasing their unpredictable nature.
Spin and Magnetic Moment: Uncovering Intrinsic Properties and their Significance
Electron also possess “spin,” an intrinsic property that gives rise to their magnetic moment. Spin can be thought of as a tiny magnet within the electron, influencing how they interact with magnetic fields. This property is essential for technologies like MRI and quantum computing.
Electron Behavior in Atoms
Atomic Structure and Electron Orbitals: The Bohr Model and its Limitations
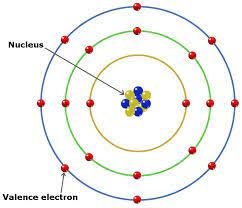
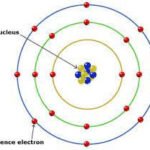
Electron orbit the nucleus of an atom in specific paths. The Bohr model describes these paths as orbitals, visualized as fixed orbits. However, this model has limitations, particularly when explaining more complex atoms.
Quantum Mechanics and Electron Clouds: A More Accurate Description of Electron Location
Quantum mechanics offers a better understanding of where electrons are. Instead of fixed orbits, electrons exist in “clouds” that represent probabilities. This means we can’t pinpoint their location but can predict where they are likely to be found.
Electron Configuration and the Periodic Table: Understanding Chemical Properties Through Electron Arrangement
The arrangement of electron, known as electron configuration, plays a vital role in determining an element’s chemical properties. The periodic table groups elements based on their electron configurations, illustrating the connection between electron arrangement and reactive behavior.
Electron Behavior in Materials
Conductors, Insulators, and Semiconductors: Electron Mobility and Material Properties
Materials can be categorized by how well they allow electrons to move.
- Conductors (e.g., copper) readily allow electron flow.
- Insulators (e.g., rubber) restrict electron movement.
- Semiconductors (e.g., silicon) fall between the two, enabling controlled flow of electrons.
Band Theory and Energy Levels: Explaining Material Behavior Based on Electron States
Band theory explains how electrons fill energy levels in materials. In conductors, energy levels overlap, allowing free movement of electrons. In insulators, the gap between energy levels is too wide, preventing flow. Semiconductors have a moderate gap, which can be manipulated through doping or applying voltages.
Practical Applications: The Role of Electrons in Modern Electronics
Electron are at the heart of modern electronics. Transistors, diodes, and microchips rely on controlled electron movement. Advancements in technology owe much to our understanding of how electrons behave in different materials.
The Electron in Chemical Reactions
Oxidation and Reduction: Electron Transfer and Chemical Bonding
Electron are crucial in chemical reactions, particularly in oxidation and reduction processes. Oxidation involves losing electrons, while reduction means gaining them. These transfers are fundamental to how chemical bonds form and break.
Ionic and Covalent Bonding: Understanding Different Types of Chemical Bonds
There are two main types of bonding influenced by electrons:
- Ionic bonds occur when electrons transfer between atoms, creating charged ions.
- Covalent bonds involve the sharing of electrons between atoms.
These bonds determine the properties and behavior of compounds.
Real-World Examples: Everyday Chemistry Explained Through Electron Interactions
A common example of electron interaction is rust formation. When iron reacts with oxygen and moisture, it undergoes oxidation, losing electrons and forming iron oxide, or rust.
The Electron and Beyond: Current Research and Future Directions
Quantum Computing and Electron Spin: Exploring the Potential of Spintronics
Research in quantum computing is harnessing electron spin. This area, known as spintronics, seeks to use the spin of electrons for information processing, potentially revolutionizing computing power.
Advanced Materials Science and Electron Manipulation: Nanotechnology and its Applications
Scientists are exploring how to manipulate electrons on an atomic level. Nanotechnology, which deals with materials at the nanoscale, has vast potential in medicine, electronics, and energy.
Open Questions and Ongoing Research in Electron Physics: Areas for Future Investigation
Many questions remain about electrons. Researchers are investigating phenomena like electron entanglement and how they could impact communication technologies and understanding the universe.
Conclusion: Key Takeaways and the Enduring Significance of Electron Theory
Electron theory is a cornerstone of modern science, impacting various fields such as physics, chemistry, and engineering.
- Understanding electrons helps us grasp material properties.
- It explains chemical reactions and bonding.
- The ongoing research in electron science promises exciting advancements.
Exploring electron theory offers insights into the workings of the universe and inspires future innovations. Embrace the wonders of electron physics and consider how these invisible particles shape the world around us. view images.